Sperm

Sperm (pl.: sperm or sperms) is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction (forms in which there is a larger, female reproductive cell and a smaller, male one). Animals produce motile sperm with a tail known as a flagellum, which are known as spermatozoa, while some red algae and fungi produce non-motile sperm cells, known as spermatia.[1] Flowering plants contain non-motile sperm inside pollen, while some more basal plants like ferns and some gymnosperms have motile sperm.[2]
Sperm cells form during the process known as spermatogenesis, which in amniotes (reptiles and mammals) takes place in the seminiferous tubules of the testicles.[3] This process involves the production of several successive sperm cell precursors, starting with spermatogonia, which differentiate into spermatocytes. The spermatocytes then undergo meiosis, reducing their chromosome number by half, which produces spermatids. The spermatids then mature and, in animals, construct a tail, or flagellum, which gives rise to the mature, motile sperm cell. This whole process occurs constantly and takes around 3 months from start to finish.
Sperm cells cannot divide and have a limited lifespan, but after fusion with egg cells during fertilization, a new organism begins developing, starting as a totipotent zygote. The human sperm cell is haploid, so that its 23 chromosomes can join the 23 chromosomes of the female egg to form a diploid cell with 46 paired chromosomes. In mammals, sperm is stored in the epididymis and released through the penis in semen during ejaculation.
The word sperm is derived from the Greek word σπέρμα, sperma, meaning "seed".
Evolution
It is generally accepted that isogamy is the ancestor to sperm and eggs. Because there are no fossil records of the evolution of sperm and eggs from isogamy, there is a strong emphasis on mathematical models to understand the evolution of sperm.[4]
A widespread hypothesis states that sperm evolved rapidly, but there is no direct evidence that sperm evolved at a fast rate or before other male characteristics.[5]
Sperm in animals
Function
The main sperm function is to reach the ovum and fuse with it to deliver two sub-cellular structures: (i) the male pronucleus that contains the genetic material and (ii) the centrioles that are structures that help organize the microtubule cytoskeleton.[clarification needed]
The nuclear DNA in sperm cells is haploid, that is, they contribute only one copy of each paternal chromosome pair. Mitochondria in human sperm contain no or very little DNA because mtDNA is degraded while sperm cells are maturing, hence they typically do not contribute any genetic material to their offspring.[6]
Anatomy
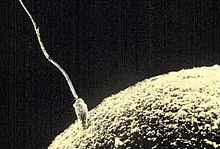
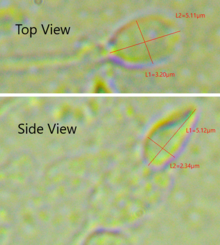
The mammalian sperm cell can be divided in 2 parts connected by a neck:
- Head: contains the nucleus with densely coiled chromatin fibers, surrounded anteriorly by a thin, flattened sac called the acrosome, which contains enzymes used for penetrating the female egg. It also contains vacuoles.[7]
- Tail: also called the flagellum, is the longest part and capable of wave-like motion that propels sperm for swimming and aids in the penetration of the egg.[8][9][10] The tail was formerly thought to move symmetrically in a helical shape.
- Neck: also called connecting piece contains one typical centriole and one atypical centriole such as the proximal centriole-like.[11][12] The midpiece has a central filamentous core with many mitochondria spiralled around it, used for ATP production for the journey through the female cervix, uterus, and oviducts.
During fertilization, the sperm provides three essential parts to the oocyte: (1) a signalling or activating factor, which causes the metabolically dormant oocyte to activate; (2) the haploid paternal genome; (3) the centriole, which is responsible for forming the centrosome and microtubule system.[13]
Origin
The spermatozoa of animals are produced through spermatogenesis inside the male gonads (testicles) via meiotic division. The initial spermatozoon process takes around 70 days to complete. The process starts with the production of spermatogonia from germ cell precursors. These divide and differentiate into spermatocytes, which undergo meiosis to form spermatids. In the spermatid stage, the sperm develops the familiar tail. The next stage where it becomes fully mature takes around 60 days when it is called a spermatozoan.[14] Sperm cells are carried out of the male body in a fluid known as semen. Human sperm cells can survive within the female reproductive tract for more than 5 days post coitus.[15] Semen is produced in the seminal vesicles, prostate gland and urethral glands.
In 2016, scientists at Nanjing Medical University claimed they had produced cells resembling mouse spermatids from mouse embryonic stem cells artificially. They injected these spermatids into mouse eggs and produced pups.[16]
Sperm quality
Sperm quantity and quality are the main parameters in semen quality, which is a measure of the ability of semen to accomplish fertilization. Thus, in humans, it is a measure of fertility in a man. The genetic quality of sperm, as well as its volume and motility, all typically decrease with age.[17] DNA double-strand breaks in sperm increase with age.[18] Also apoptosis decreases with age suggesting that the increase in damaged DNA of sperm as men age occurs partly as a result of less efficient cell selection (apoptosis) operating during or after spermatogenesis.[18]
DNA damages present in sperm cells in the period after meiosis but before fertilization may be repaired in the fertilized egg, but if not repaired, can have serious deleterious effects on fertility and the developing embryo. Human sperm cells are particularly vulnerable to free radical attack and the generation of oxidative DNA damage,[19] such as that from 8-Oxo-2'-deoxyguanosine.
The postmeiotic phase of mouse spermatogenesis is very sensitive to environmental genotoxic agents, because as male germ cells form mature sperm they progressively lose the ability to repair DNA damage.[20] Irradiation of male mice during late spermatogenesis can induce damage that persists for at least 7 days in the fertilizing sperm cells, and disruption of maternal DNA double-strand break repair pathways increases sperm cell-derived chromosomal aberrations.[21] Treatment of male mice with melphalan, a bifunctional alkylating agent frequently employed in chemotherapy, induces DNA lesions during meiosis that may persist in an unrepaired state as germ cells progress through DNA repair-competent phases of spermatogenic development.[22] Such unrepaired DNA damages in sperm cells, after fertilization, can lead to offspring with various abnormalities.
Sperm size
Related to sperm quality is sperm size, at least in some animals. For instance, the sperm of some species of fruit fly (Drosophila) are up to 5.8 cm long—about 20 times as long as the fly itself. Longer sperm cells are better than their shorter counterparts at displacing competitors from the female's seminal receptacle. The benefit to females is that only healthy males carry "good" genes that can produce long sperm in sufficient quantities to outcompete their competitors.[23][24]
Market for human sperm
Some sperm banks hold up to 170 litres (37 imp gal; 45 US gal) of sperm.[25]
In addition to ejaculation, it is possible to extract sperm through testicular sperm extraction.
On the global market, Denmark has a well-developed system of human sperm export. This success mainly comes from the reputation of Danish sperm donors for being of high quality[26] and, in contrast with the law in the other Nordic countries, gives donors the choice of being either anonymous or non-anonymous to the receiving couple.[26] Furthermore, Nordic sperm donors tend to be tall and highly educated[27] and have altruistic motives for their donations,[27] partly due to the relatively low monetary compensation in Nordic countries. More than 50 countries worldwide are importers of Danish sperm, including Paraguay, Canada, Kenya, and Hong Kong.[26] However, the Food and Drug Administration (FDA) of the US has banned import of any sperm, motivated by a risk of transmission of Creutzfeldt–Jakob disease, although such a risk is insignificant, since artificial insemination is very different from the route of transmission of Creutzfeldt–Jakob disease.[28] The prevalence of Creutzfeldt–Jakob disease for donors is at most one in a million, and if the donor was a carrier, the infectious proteins would still have to cross the blood-testis barrier to make transmission possible.[28]
History
Sperm were first observed in 1677 by Antonie van Leeuwenhoek[29] using a microscope. He described them as being animalcules (little animals), probably due to his belief in preformationism, which thought that each sperm contained a fully formed but small human.[citation needed]
Forensic analysis
Ejaculated fluids are detected by ultraviolet light, irrespective of the structure or colour of the surface.[30] Sperm heads, e.g. from vaginal swabs, are still detected by microscopy using the "Christmas Tree Stain" method, i.e., Kernechtrot-Picroindigocarmine (KPIC) staining.[31][32]
Sperm in plants
Sperm cells in algal and many plant gametophytes are produced in male gametangia (antheridia) via mitotic division. In flowering plants, sperm nuclei are produced inside pollen.[33]
Motile sperm cells

Motile sperm cells typically move via flagella and require a water medium in order to swim toward the egg for fertilization. In animals most of the energy for sperm motility is derived from the metabolism of fructose carried in the seminal fluid. This takes place in the mitochondria located in the sperm's midpiece (at the base of the sperm head). These cells cannot swim backwards due to the nature of their propulsion. The uniflagellated sperm cells (with one flagellum) of animals are referred to as spermatozoa, and are known to vary in size.[citation needed]
Motile sperm are also produced by many protists and the gametophytes of bryophytes, ferns and some gymnosperms such as cycads and ginkgo. The sperm cells are the only flagellated cells in the life cycle of these plants. In many ferns and lycophytes, cycads and ginkgo they are multi-flagellated (carrying more than one flagellum).[34]
In nematodes, the sperm cells are amoeboid and crawl, rather than swim, towards the egg cell.[35]
Non-motile sperm cells
Non-motile sperm cells called spermatia lack flagella and therefore cannot swim. Spermatia are produced in a spermatangium.[34]
Because spermatia cannot swim, they depend on their environment to carry them to the egg cell. Some red algae, such as Polysiphonia, produce non-motile spermatia that are spread by water currents after their release.[34] The spermatia of rust fungi are covered with a sticky substance. They are produced in flask-shaped structures containing nectar, which attract flies that transfer the spermatia to nearby hyphae for fertilization in a mechanism similar to insect pollination in flowering plants.[36]
Fungal spermatia (also called pycniospores, especially in the Uredinales) may be confused with conidia. Conidia are spores that germinate independently of fertilization, whereas spermatia are gametes that are required for fertilization. In some fungi, such as Neurospora crassa, spermatia are identical to microconidia as they can perform both functions of fertilization as well as giving rise to new organisms without fertilization.[37]
Sperm nuclei
In almost all embryophytes, including most gymnosperms and all angiosperms, the male gametophytes (pollen grains) are the primary mode of dispersal, for example via wind or insect pollination, eliminating the need for water to bridge the gap between male and female. Each pollen grain contains a spermatogenous (generative) cell. Once the pollen lands on the stigma of a receptive flower, it germinates and starts growing a pollen tube through the carpel. Before the tube reaches the ovule, the nucleus of the generative cell in the pollen grain divides and gives rise to two sperm nuclei, which are then discharged through the tube into the ovule for fertilization.[34]
In some protists, fertilization also involves sperm nuclei, rather than cells, migrating toward the egg cell through a fertilization tube. Oomycetes form sperm nuclei in a syncytical antheridium surrounding the egg cells. The sperm nuclei reach the eggs through fertilization tubes, similar to the pollen tube mechanism in plants.[34]
Sperm centrioles
Most sperm cells have centrioles in the sperm neck.[38] Sperm of many animals has two typical centrioles, known as the proximal centriole and distal centriole. Some animals (including humans and bovines) have a single typical centriole, the proximal centriole, as well as a second centriole with atypical structure.[11] Mice and rats have no recognizable sperm centrioles. The fruit fly Drosophila melanogaster has a single centriole and an atypical centriole named the proximal centriole-like.[39]
Sperm tail formation
The sperm tail is a specialized type of cilium (aka flagella). In many animals the sperm tail is formed through the unique process of cytosolic ciliogenesis, in which all or part of the sperm tail's axoneme is formed in the cytoplasm or gets exposed to the cytoplasm.[40]
See also
- List of distinct cell types in the adult human body
- Female sperm
- Female sperm storage
- Mendelian inheritance
- Polyspermy
- Sperm competition
- Sperm granuloma
- Sperm theft
Citations
- ^ "Spermatium definition and meaning". Collins English Dictionary. Retrieved 2020-02-20.
- ^ Kumar, Anil (2006). Botany for Degree Gymnosperm (Multicolor ed.). S. Chand Publishing. p. 261. ISBN 978-81-219-2618-8.
- ^ "Animal reproductive system - Male systems". Encyclopedia Britannica. Retrieved 2020-02-20.
- ^ Pitnick, Scott S.; Hosken, Dave J.; Birkhead, Tim R. (2008-11-21). Sperm Biology: An Evolutionary Perspective. Academic Press. pp. 43–44. ISBN 978-0-08-091987-4.
- ^ Fitzpatrick, John L.; Bridge, C. Daisy; Snook, Rhonda R. (2020-08-12). "Repeated evidence that the accelerated evolution of sperm is associated with their fertilization function". Proceedings of the Royal Society B: Biological Sciences. 287 (1932): 20201286. doi:10.1098/rspb.2020.1286. PMC 7575512. PMID 32752988.
- ^ Lee, William; Zamudio-Ochoa, Angelica; Buchel, Gina; Podlesniy, Petar; Marti Gutierrez, Nuria; Puigròs, Margalida; Calderon, Anna; Tang, Hsin-Yao; Li, Li; Mikhalchenko, Aleksei; Koski, Amy; Trullas, Ramon; Mitalipov, Shoukhrat; Temiakov, Dmitry (October 2023). "Molecular basis for maternal inheritance of human mitochondrial DNA". Nature Genetics. 55 (10): 1632–1639. doi:10.1038/s41588-023-01505-9. ISSN 1546-1718. PMC 10763495. PMID 37723262.
- ^ Boitrelle, F; Guthauser, B; Alter, L; Bailly, M; Wainer, R; Vialard, F; Albert, M; Selva, J (2013). "The nature of human sperm head vacuoles: a systematic literature review". Basic Clin Androl. 23: 3. doi:10.1186/2051-4190-23-3. PMC 4346294. PMID 25780567.
- ^ Fawcett, D. W. (1981) Sperm Flagellum. In: The Cell. D. W. Fawcett. Philadelphia, W. B. Saunders Company. 14: pp. 604-640.
- ^ Lehti, M. S. and A. Sironen (2017). "Formation and function of sperm tail structures in association with sperm motility defects." Bi
- ^ Ishijima, Sumio; Oshio, Shigeru; Mohri, Hideo (1986). "Flagellar movement of human spermatozoa". Gamete Research. 13 (3): 185–197. doi:10.1002/mrd.1120130302.
- ^ a b Fishman, Emily L; Jo, Kyoung; Nguyen, Quynh P. H; Kong, Dong; Royfman, Rachel; Cekic, Anthony R; Khanal, Sushil; Miller, Ann L; Simerly, Calvin; Schatten, Gerald; Loncarek, Jadranka; Mennella, Vito; Avidor-Reiss, Tomer (2018). "A novel atypical sperm centriole is functional during human fertilization". Nature Communications. 9 (1): 2210. Bibcode:2018NatCo...9.2210F. doi:10.1038/s41467-018-04678-8. PMC 5992222. PMID 29880810.
- ^ Blachon, S; Cai, X; Roberts, K. A; Yang, K; Polyanovsky, A; Church, A; Avidor-Reiss, T (2009). "A Proximal Centriole-Like Structure is Present in Drosophila Spermatids and Can Serve as a Model to Study Centriole Duplication". Genetics. 182 (1): 133–44. doi:10.1534/genetics.109.101709. PMC 2674812. PMID 19293139.
- ^ Hewitson, Laura & Schatten, Gerald P. (2003). "The biology of fertilization in humans". In Patrizio, Pasquale; et al. (eds.). A color atlas for human assisted reproduction: laboratory and clinical insights. Lippincott Williams & Wilkins. p. 3. ISBN 978-0-7817-3769-2. Retrieved 2013-11-09.
- ^ "Semen and sperm quality". Archived from the original on 2000-11-10. Retrieved 2011-04-10.
- ^ Gould, JE; Overstreet, JW; Hanson, FW (1984). "Assessment of human sperm function after recovery from the female reproductive tract". Biology of Reproduction. 31 (5): 888–894. doi:10.1095/biolreprod31.5.888. PMID 6518230.
- ^ Cyranoski, David (2016). "Researchers claim to have made artificial mouse sperm in a dish". Nature. doi:10.1038/nature.2016.19453. S2CID 87014225.
- ^ Gurevich, Rachel (2008-06-10). "Does Age Affect Male Fertility?". About.com. Archived from the original on 2015-09-28. Retrieved 14 February 2010.
- ^ a b Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003 Dec;80(6):1420-30. doi: 10.1016/j.fertnstert.2003.04.002. PMID: 14667878
- ^ Gavriliouk D, Aitken RJ (2015). "Damage to Sperm DNA Mediated by Reactive Oxygen Species: Its Impact on Human Reproduction and the Health Trajectory of Offspring". The Male Role in Pregnancy Loss and Embryo Implantation Failure. Advances in Experimental Medicine and Biology. Vol. 868. pp. 23–47. doi:10.1007/978-3-319-18881-2_2. ISBN 978-3-319-18880-5. PMID 26178844.
- ^ Marchetti F, Wyrobek AJ (2008). "DNA repair decline during mouse spermiogenesis results in the accumulation of heritable DNA damage" (PDF). DNA Repair. 7 (4): 572–81. doi:10.1016/j.dnarep.2007.12.011. PMID 18282746. S2CID 1316244.
- ^ Marchetti F, Essers J, Kanaar R, Wyrobek AJ (2007). "Disruption of maternal DNA repair increases sperm-derived chromosomal aberrations". Proceedings of the National Academy of Sciences of the United States of America. 104 (45): 17725–9. Bibcode:2007PNAS..10417725M. doi:10.1073/pnas.0705257104. PMC 2077046. PMID 17978187.
- ^ Marchetti F, Bishop J, Gingerich J, Wyrobek AJ (2015). "Meiotic interstrand DNA damage escapes paternal repair and causes chromosomal aberrations in the zygote by maternal misrepair". Scientific Reports. 5: 7689. Bibcode:2015NatSR...5E7689M. doi:10.1038/srep07689. PMC 4286742. PMID 25567288.
- ^ Lüpold, Stefan; Manier, Mollie K; Puniamoorthy, Nalini; Schoff, Christopher; Starmer, William T; Luepold, Shannon H. Buckley; Belote, John M; Pitnick, Scott (2016). "How sexual selection can drive the evolution of costly sperm ornamentation". Nature. 533 (7604): 535–8. Bibcode:2016Natur.533..535L. doi:10.1038/nature18005. PMID 27225128. S2CID 4407752.
- ^ Gardiner, Jennifer R (2016). "The bigger, the better". Nature. 533 (7604): 476. doi:10.1038/533476a. PMID 27225117.
- ^ Sarfraz Manzoor (2 November 2012). "Come inside: the world's biggest sperm bank". The Guardian. Retrieved 4 August 2013.
- ^ a b c Assisted Reproduction in the Nordic Countries Archived 2013-11-11 at the Wayback Machine ncbio.org
- ^ a b FDA Rules Block Import of Prized Danish Sperm Posted Aug 13, 08 7:37 AM CDT in World, Science & Health
- ^ a b Steven Kotler (26 September 2007). "The God of Sperm".
- ^ "Timeline: Assisted reproduction and birth control". CBC News. Retrieved 2006-04-06.
- ^ Fiedler, Anja; Rehdorf, Jessica; Hilbers, Florian; Johrdan, Lena; Stribl, Carola; Benecke, Mark (2008). "Detection of Semen (Human and Boar) and Saliva on Fabrics by a Very High Powered UV-/VIS-Light Source". The Open Forensic Science Journal. 1: 12–15. doi:10.2174/1874402800801010012.
- ^ Allery, J. P; Telmon, N; Mieusset, R; Blanc, A; Rougé, D (2001). "Cytological detection of spermatozoa: Comparison of three staining methods". Journal of Forensic Sciences. 46 (2): 349–51. doi:10.1520/JFS14970J. PMID 11305439.
- ^ Illinois State Police/President's DNA Initiative. "The Presidents's DNA Initiative: Semen Stain Identification: Kernechtrot" (PDF). Archived from the original (PDF) on 2016-12-26. Retrieved 2009-12-10.
- ^ Phatlane William Mokwala; Phetole Mangena (6 June 2018). Pollination in Plants. BoD – Books on Demand. p. 8. ISBN 978-1-78923-236-3.
- ^ a b c d e f Raven, Peter H.; Ray F. Evert; Susan E. Eichhorn (2005). Biology of Plants, 7th Edition. New York: W.H. Freeman and Company Publishers. ISBN 0-7167-1007-2.
- ^ Bottino D, Mogilner A, Roberts T, Stewart M, Oster G (2002). "How nematode sperm crawl". Journal of Cell Science. 115 (Pt 2): 367–84. doi:10.1242/jcs.115.2.367. PMID 11839788.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sumbali, Geeta (2005). The Fungi. Alpha Science Int'l Ltd. ISBN 1-84265-153-6.
- ^ Maheshwari R (1999). "Microconidia of Neurospora crassa". Fungal Genetics and Biology. 26 (1): 1–18. doi:10.1006/fgbi.1998.1103. PMID 10072316.
- ^ Avidor-Reiss, T; Khire, A; Fishman, EL; Jo, KH (2015). "Atypical centrioles during sexual reproduction". Front Cell Dev Biol. 3: 21. doi:10.3389/fcell.2015.00021. PMC 4381714. PMID 25883936.
- ^ Blachon, S.; Cai, X.; Roberts, K. A.; Yang, K.; Polyanovsky, A.; Church, A.; Avidor-Reiss, T. (May 2009). "A Proximal Centriole-Like Structure Is Present in Drosophila Spermatids and Can Serve as a Model to Study Centriole Duplication". Genetics. 182 (1): 133–44. doi:10.1534/genetics.109.101709. PMC 2674812. PMID 19293139.
- ^ Avidor-Reiss, Tomer; Leroux, Michel R (2015). "Shared and Distinct Mechanisms of Compartmentalized and Cytosolic Ciliogenesis". Current Biology. 25 (23): R1143–50. Bibcode:2015CBio...25R1143A. doi:10.1016/j.cub.2015.11.001. PMC 5857621. PMID 26654377.
General and cited sources
- Fawcett, D. W. (1981). "Sperm Flagellum". In: D. W. Fawcett. The Cell, 2nd ed (registration required). Philadelphia: W. B. Saunders Company. pp. 604–640 (registration required). ISBN 9780721635842. OCLC 993416586.
- Lehti, M. S. and A. Sironen (October 2017). "Formation and function of sperm tail structures in association with sperm motility defects". Biol Reprod 97(4): 522–536. doi:10.1093/biolre/iox096.
